Hydrogels for stem cell transplantation and drug delivery
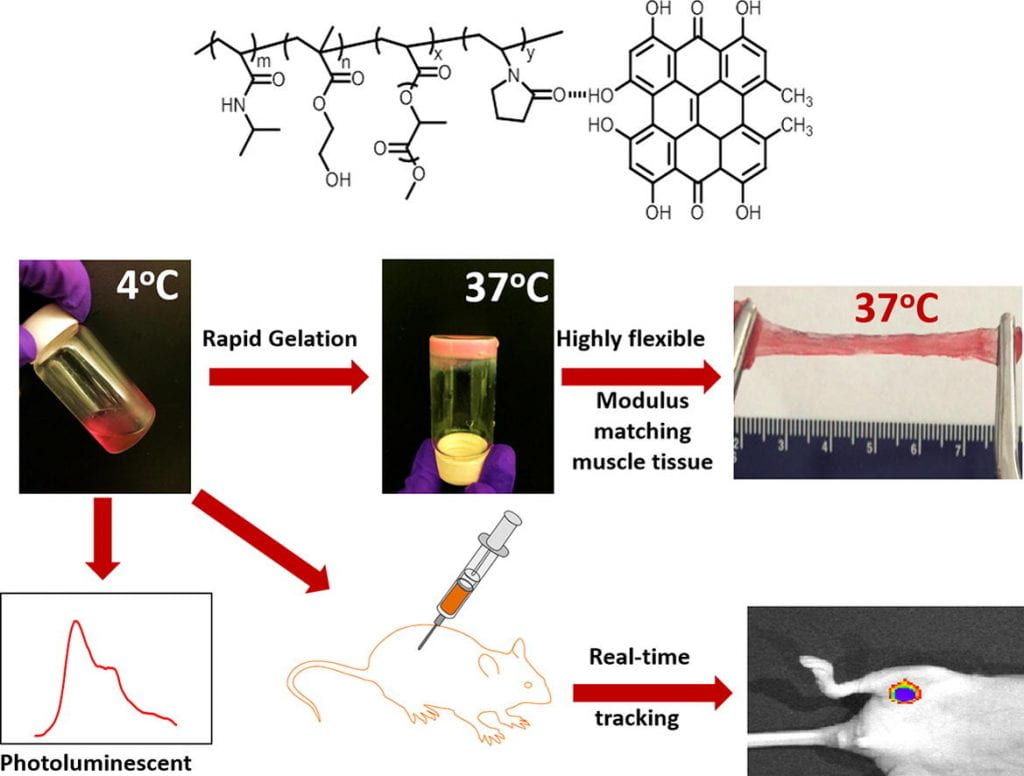
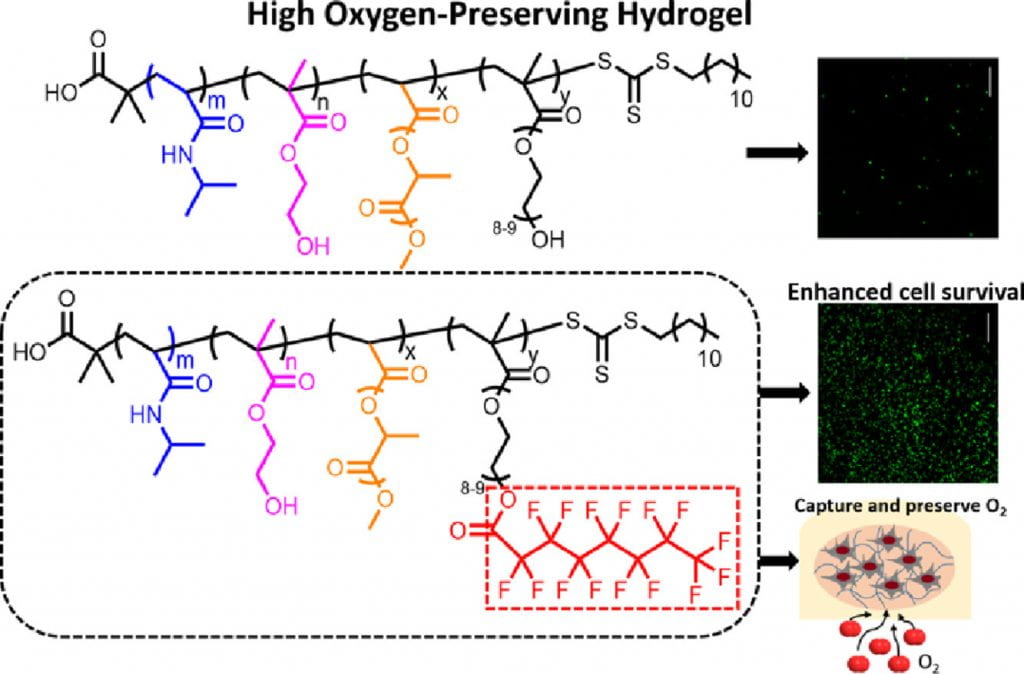
We develop highly flexible, fast gelation, thermal responsive, injectable and degradable hydrogels for stem cell transplantation and drug delivery into mechanically active soft tissues like heart and skeletal muscles. Current hydrogels are not well suited for delivery of stem cells and drugs into mechanically active soft tissues. This is mainly because (1) they have low cell and drug retention in tissues caused by slow gelation, (2) gelation process may need potentially cytotoxic chemicals, and (3) the formed gels have low flexibility and cannot maintain intact in the tissue causing burst drug release, and stem cells exposure to hostile disease environment. To address these issues, we develop highly flexible, fast gelation, thermal responsive, injectable and degradable hydrogels based on poly(N-Isopropylacryamide) copolymers. We functionalize these hydrogels to be photoluminescent, oxygen sensitive, pH sensitive, and capable of preserving oxygen. We also functionalize the hydrogels by bioinspired modification to modulate interaction of cells and matrix. In addition, we create hydrogels capable of changing properties in response to the microenvironment at different disease stages.
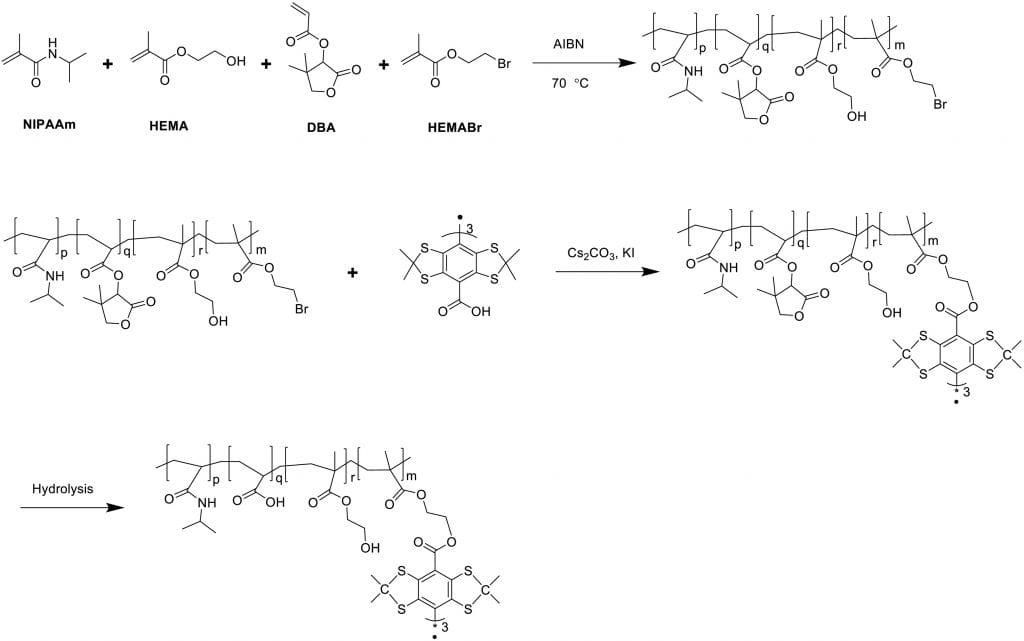
Bioimageable polymers for MRI and EPR imaging and real time monitoring of tissue oxygen content
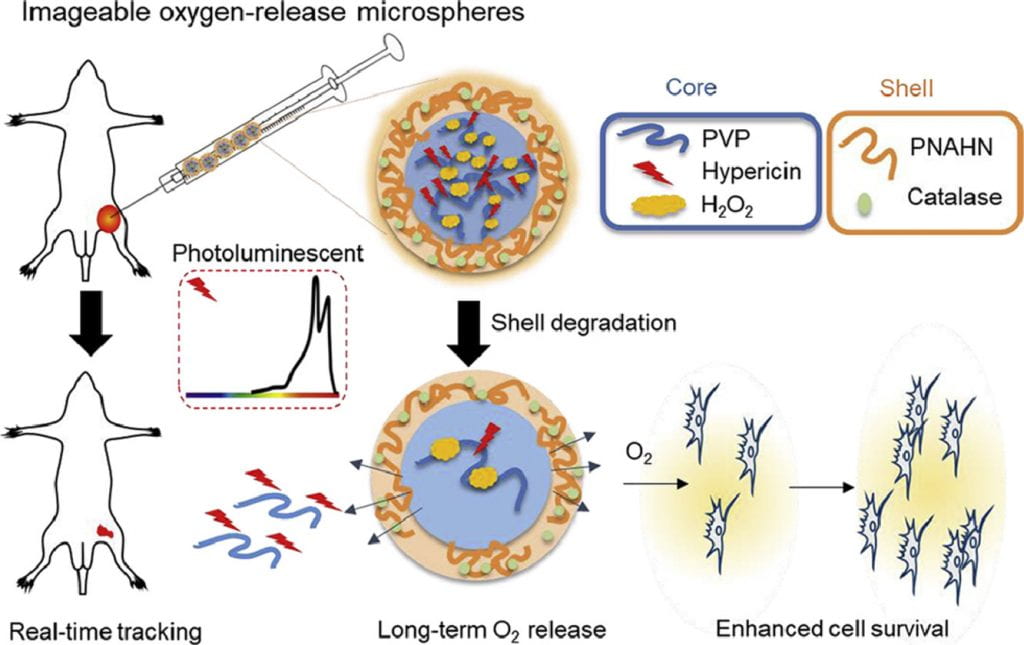
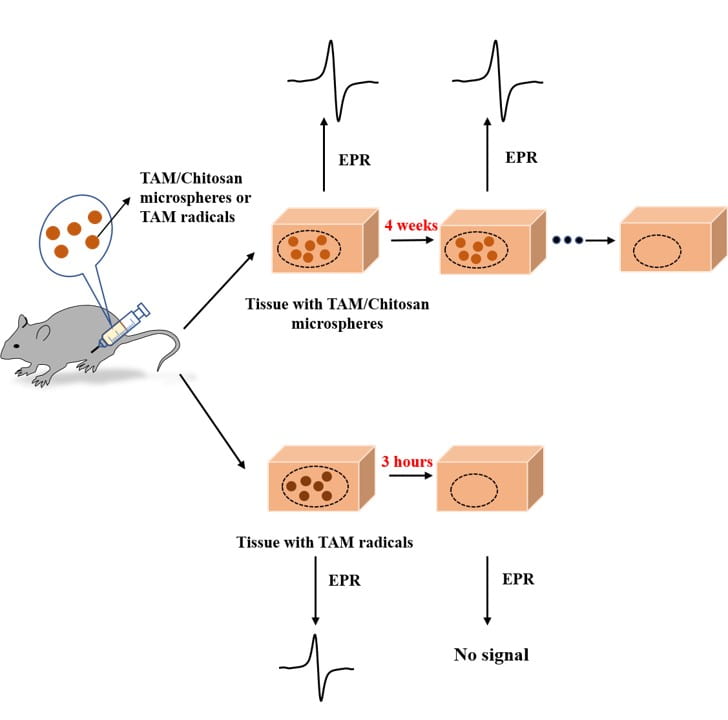
Non-invasive and real time monitoring of tissue oxygen content is highly attractive in clinics especially during tissue vascularization therapy as the therapeutic efficacy can be readily judged by tissue oxygen content. Current approaches for oxygen detection are mostly invasive. The non-invasive approaches like EPR and MRI needs repeated injection of small molecular probes, which may have toxicity concerns. There are no probes that can stay in the tissues stably for long term monitoring of tissue oxygen. We develop series of polymer-based, degradable, injectable EPR and MRI probes for long term and real time monitoring of tissue oxygen. These polymers have high oxygen permeability and sensitivity.
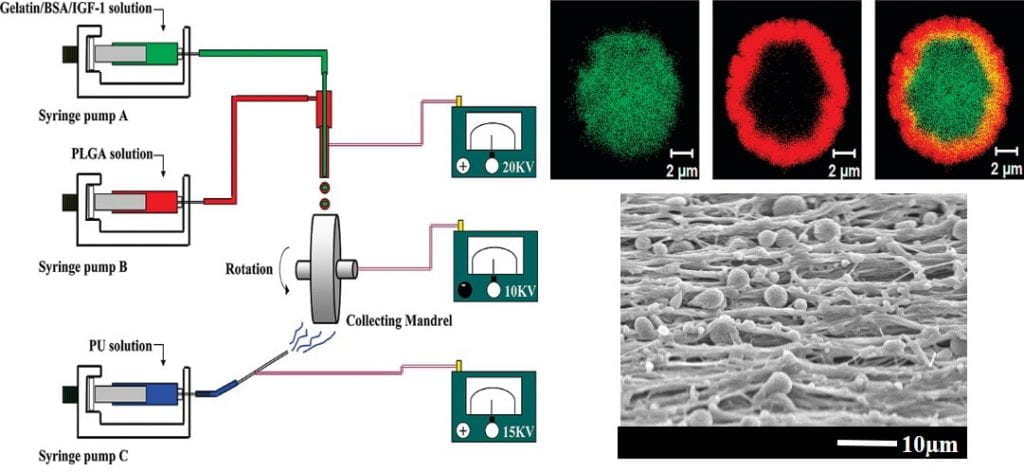
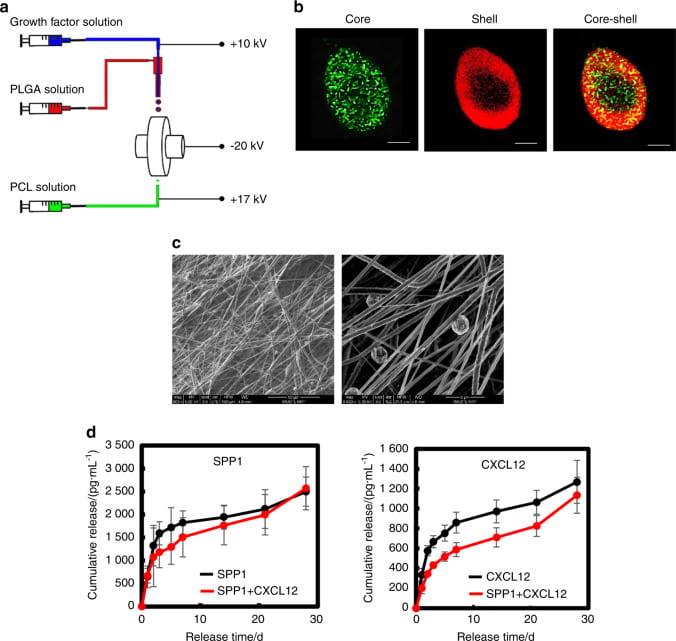
Novel processing methods to rapidly generate tissue constructs
We develop novel processing methods to rapidly generate tissue constructs that contain stem cells, growth factors and nanofibers. The tissue constructs also mimic structural properties and match mechanical properties of the target tissues. Traditional methods to fabricate tissue constructs follow a “seed and grow” approach. This is time-consuming. It remains challenging to fabricate cell populated tissue constructs in a short period of time. To facilitate tissue regeneration, tissue constructs should ideally mimic target tissue-specific properties as it may provide a native-like environment for cell growth and differentiation. We develop novel fabrication techniques capable of rapidly (~40 min) generating highly cellularized tissue constructs mimicking properties of the myocardium. The tissue constructs are assembled by simultaneously electrospraying stem cells, electrospraying growth factor-containing microspheres, and electrospinning polymer nanofibers. We create mathematical models to correlate scaffold structure, mechanical properties, and stem cell differentiation.
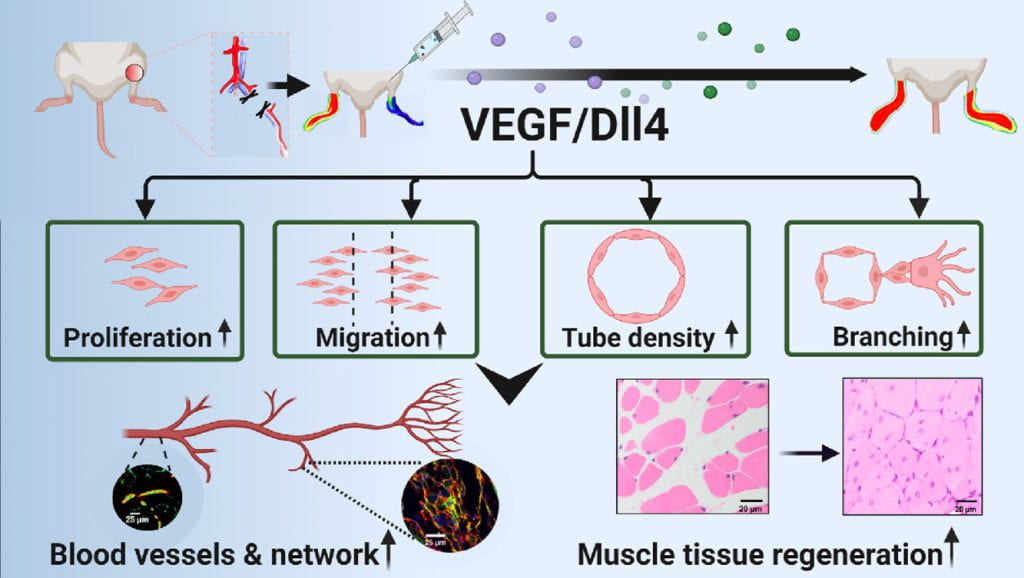
Vascularization and skeletal muscle regeneration in ischemic limbs
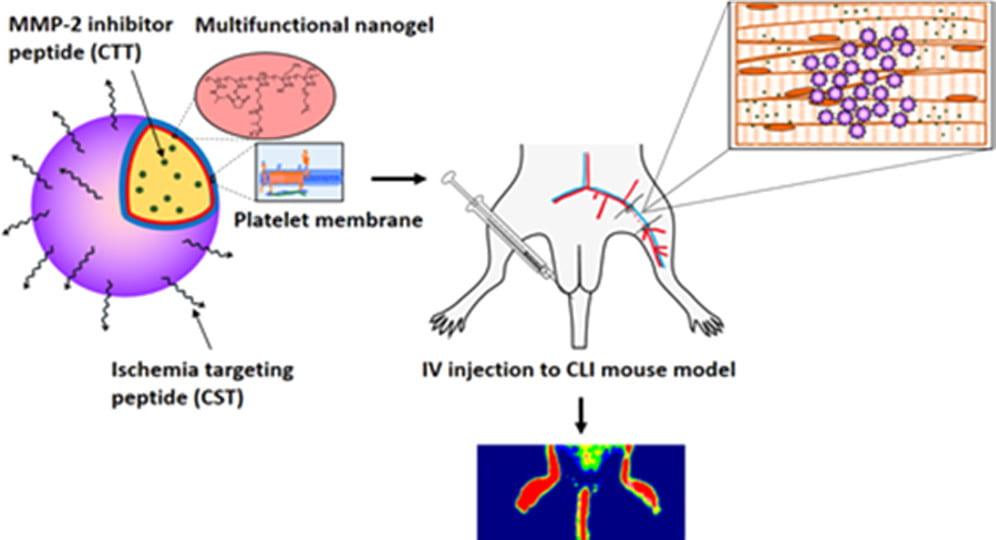
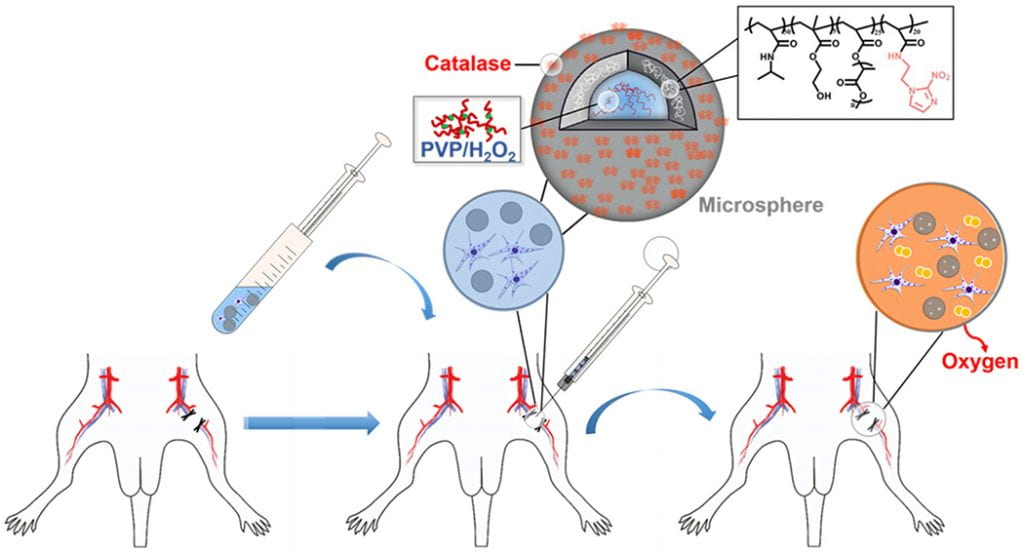
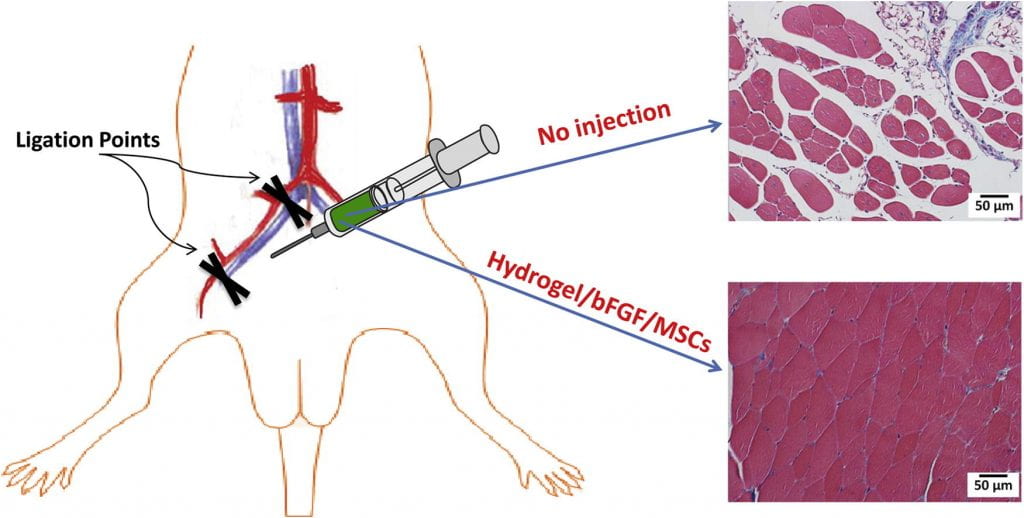
We work on vascularization and skeletal muscle regeneration in ischemic limbs using stem cell transplantation and drug delivery strategies. We create novel microenvironments in scaffolds and hydrogels to promote stem cell survival, paracrine effects and differentiation under ischemic conditions. Stem cell transplantation has low efficacy in ischemic tissues due to poor cell survival, paracrine effects and differentiation under the low nutrient, hypoxic and inflammatory conditions. Current approaches cannot effectively improve cell survival upon cell transplantation. We create prosurvival microenvironments in scaffolds and hydrogels that release oxygen, prosurvival growth factors and proangiogenic growth factors to improve cell survival. The microenvironments also promote stem cell differentiation, improve cell paracrine effects, stimulate fast angiogenesis, and enhance skeletal muscle regeneration.
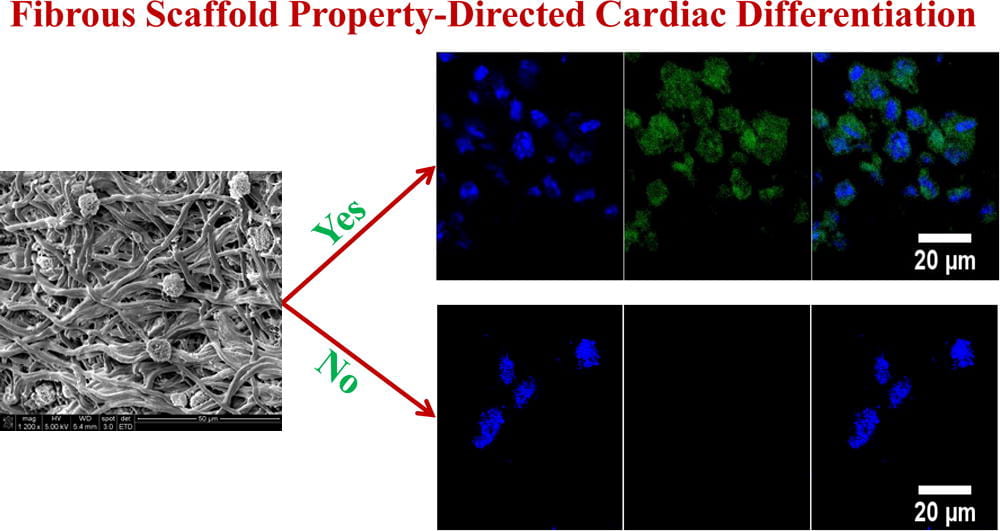
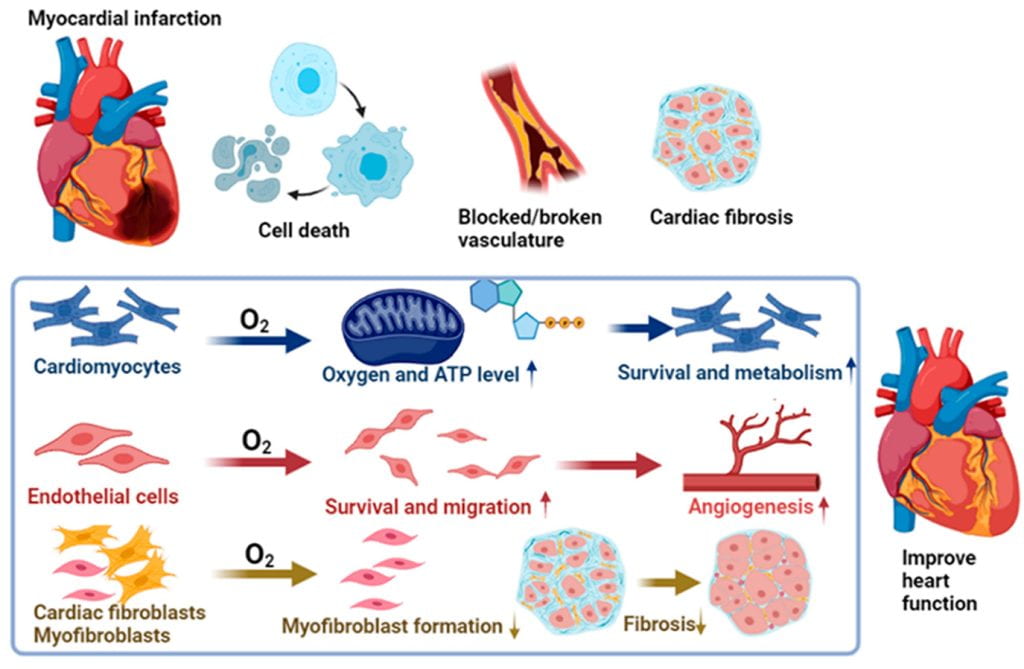
Myocardial repair following myocardial infarction and at heart failure stage
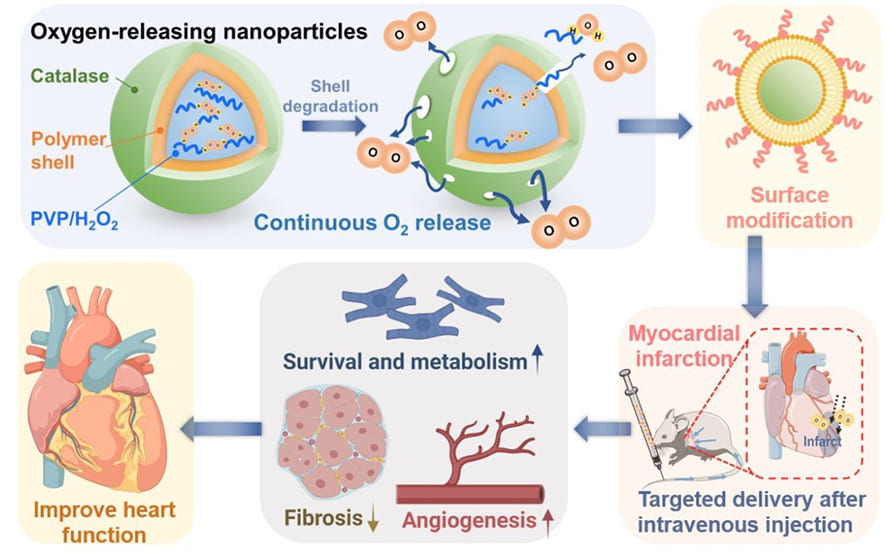
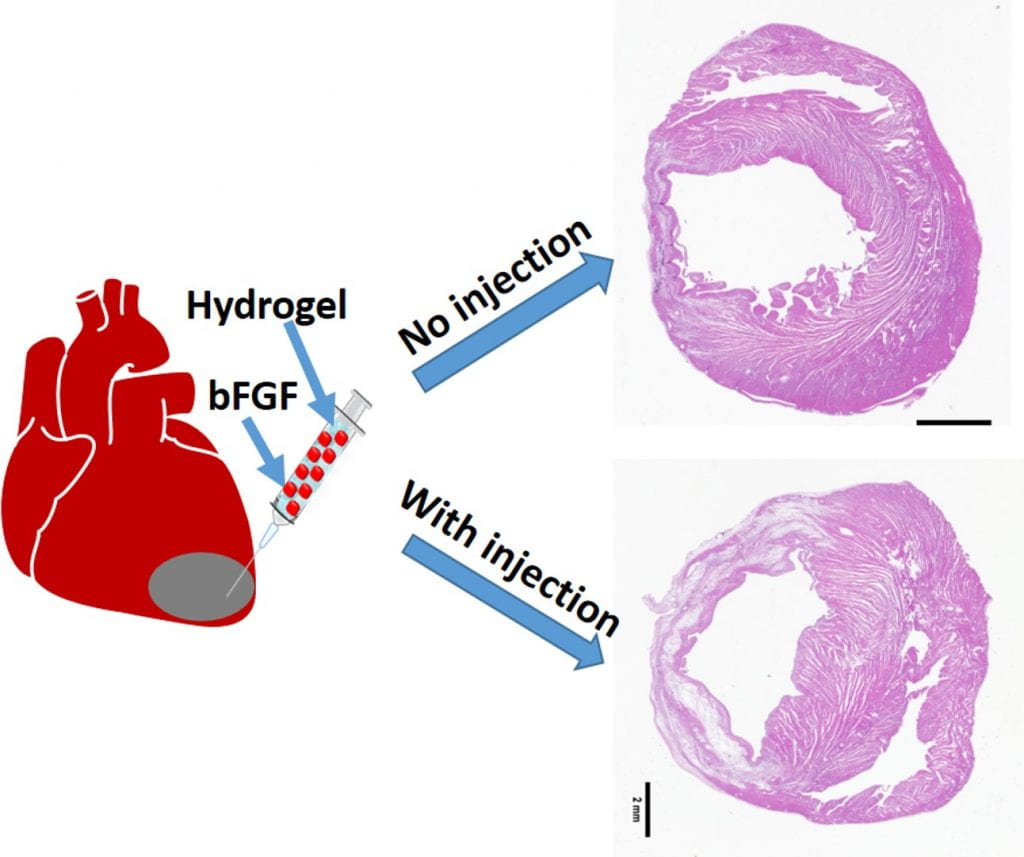
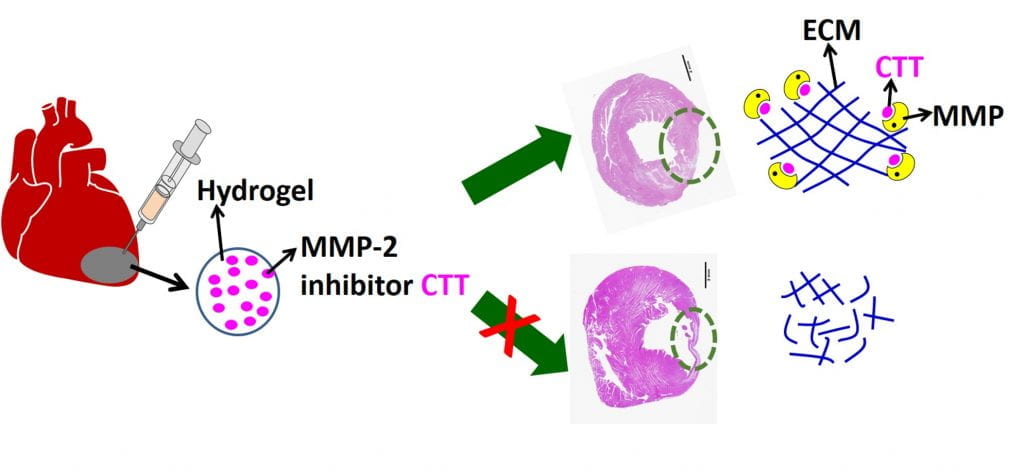
We work on myocardial repair and regeneration after myocardial infarction and at heart failure stage. We create stem cell and drug delivery systems using developed 3D scaffolds and hydrogels. The systems have the following functions: (1) direct stem cells to differentiate into cardiac lineage by using scaffolds mimicking mechanical properties and morphology of cardiac extracellular matrix (ECM); (2) increase cardiac cell survival in the ischemic environment by delivering prosurvival growth factors and oxygen; (3) preserve cardiac ECM by spatiotemporally decreasing MMP-2/9 bioactivity; (4) vascularize the cardiac ECM by delivering angiogenic growth factors; (5) attenuate cardiac fibrosis by delivering anti-fibrotic factors; and (6) control tissue inflammation by modulating macrophage phenotype and secretome.
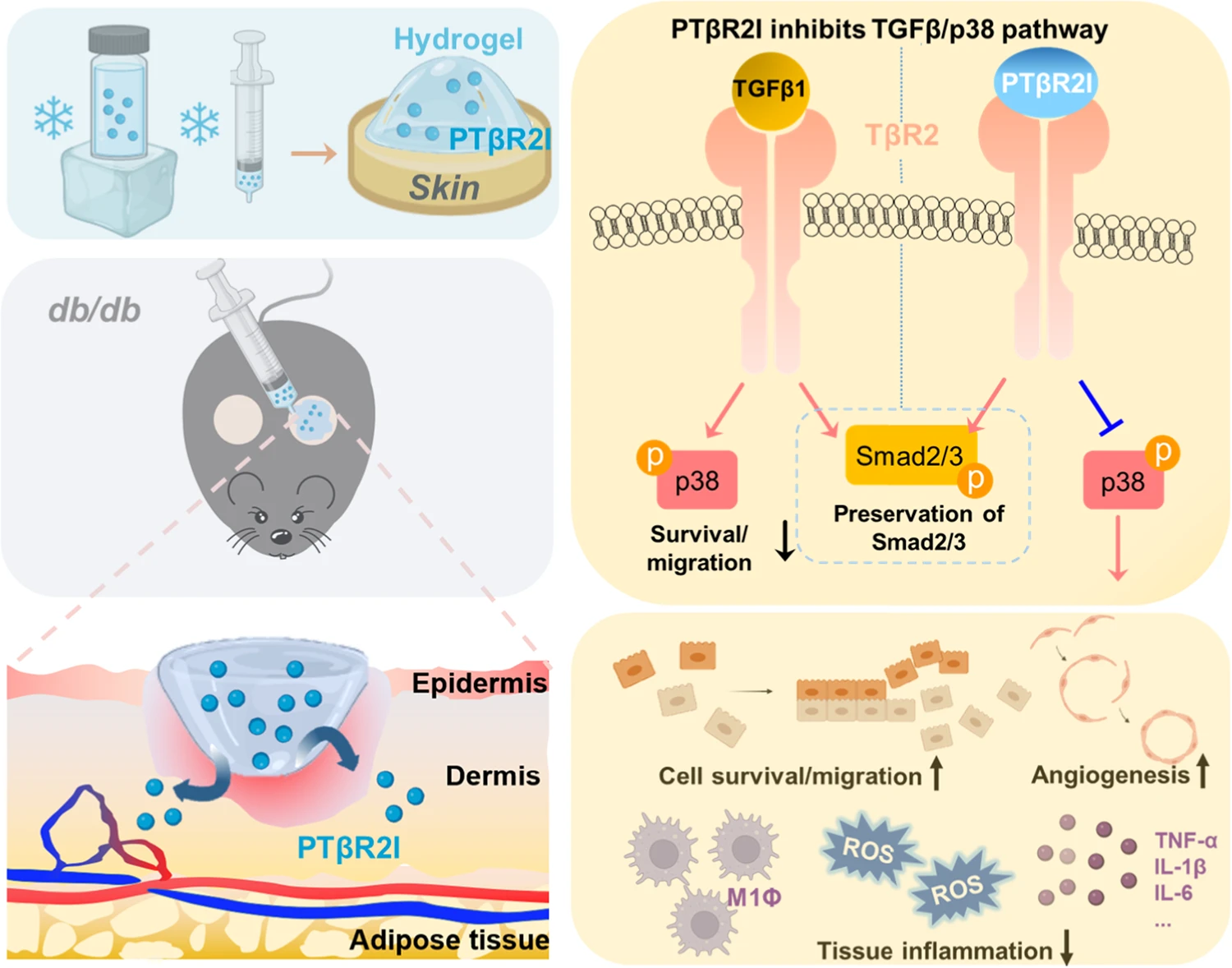
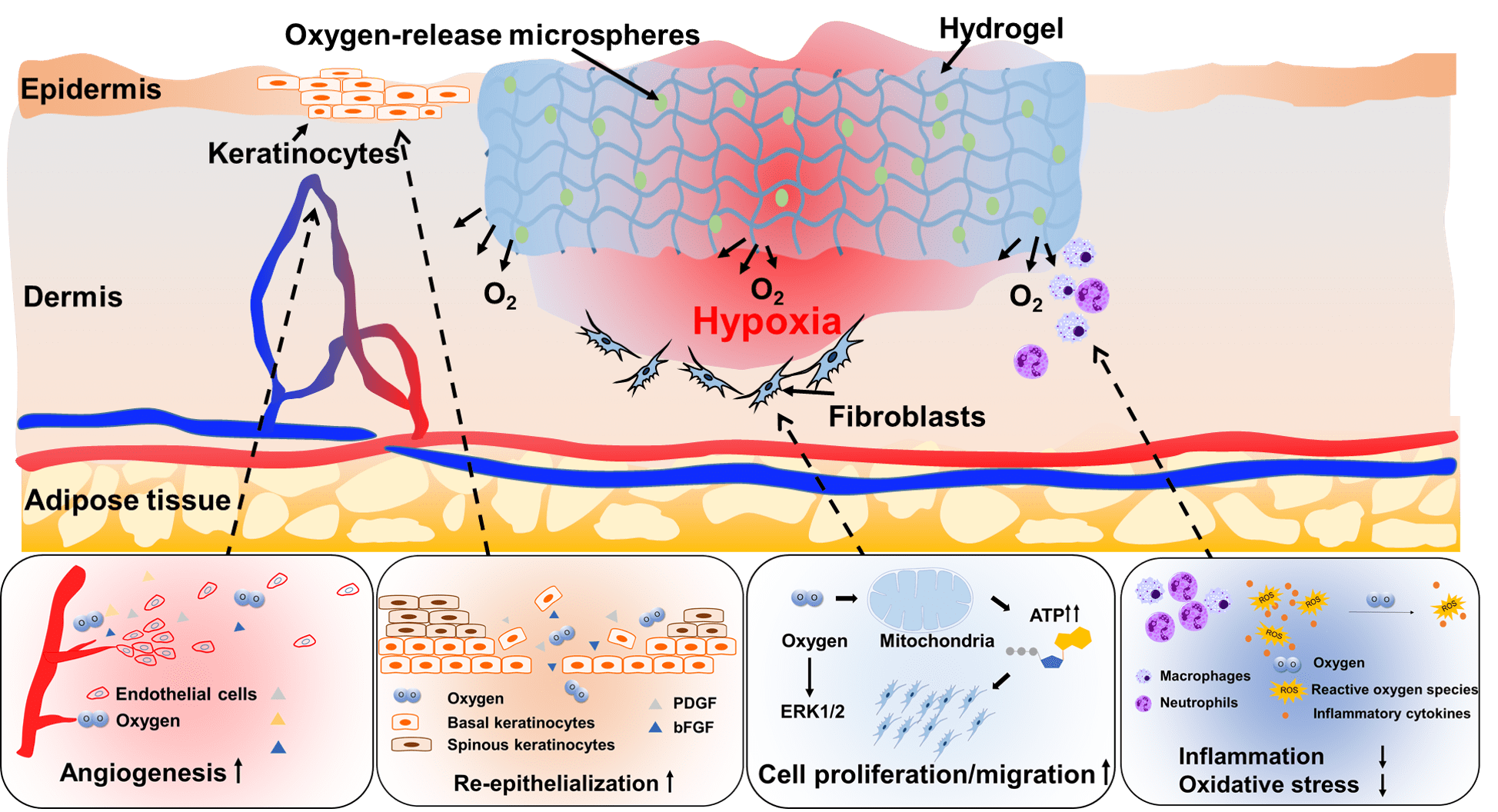
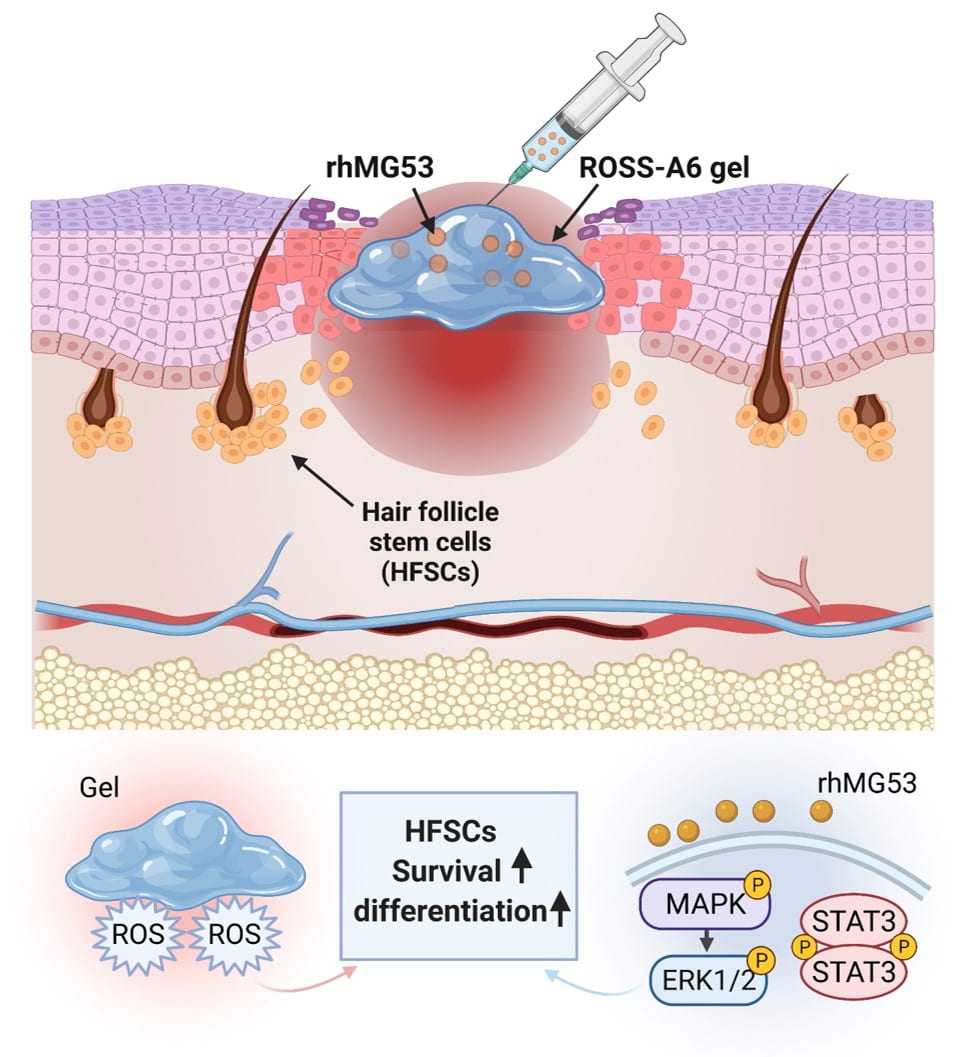
Wound healing of diabetic wounds
We and collaborators work on diabetic wound healing using stem cell transplantation and drug delivery strategies. To promote healing of diabetic wounds, we develop novel approaches including (1) sustained delivery of oxygen using a ROS-scavenging hydrogel to simultaneously promote epithelialization and angiogenesis, and decrease inflammation in the wounds; (2) controlled release of a unique protein capable of repairing cell membrane (MG53) and promoting skin cell migration; and (3) design of proteins/peptides to inhibit or upregulate signaling pathways that control wound healing.
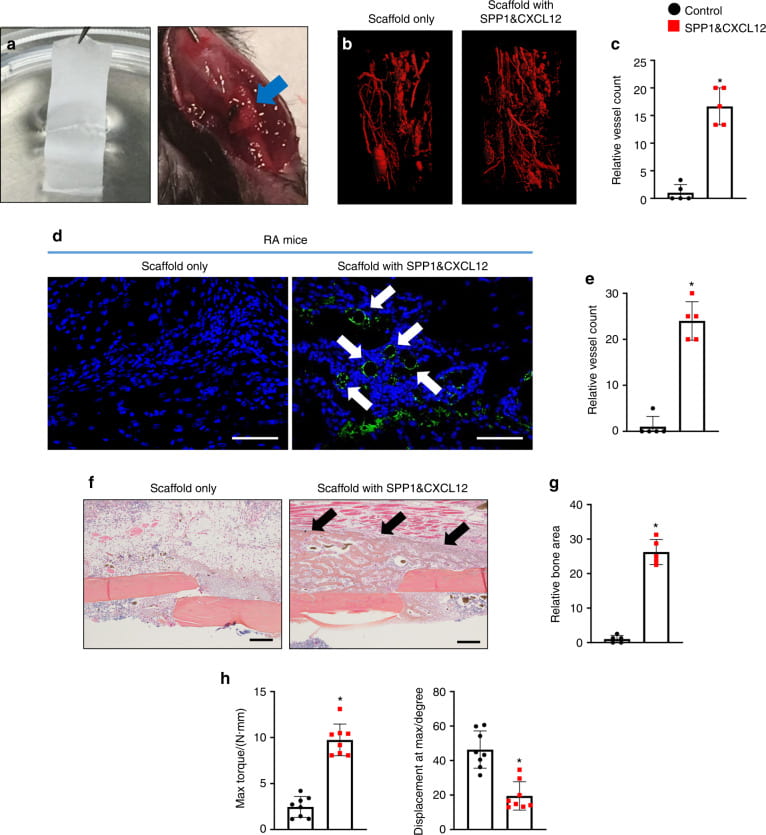
Bone regeneration
We and collaborators work on bone regeneration using facture nonunion, and critical bone defect models. We (1) create biomimetic scaffolds capable of promoting biomineralization and bone marrow stem cell proliferation; (2) fabricate scaffolds populated with engineered stem cells, and (3) develop scaffolds capable of simultaneously releasing multiple growth factors that are downregulated under inflammatory condition, to promote angiogenesis.